Talk to your doctor – could you have bronchiectasis?
To determine if you may be a candidate for vest therapy, please answer the following questions. You can share this information with your healthcare provider.
Do you currently have moderate-to-severe COPD, chronic bronchitis or bronchiectasis?
Yes
No
In the last 12 months, have you been hospitalized two or more times for respiratory issues?
Yes
No
In the last 12 months, have you needed antibiotics to treat cough/infection more than three times?
Yes
No
Have you been coughing up mucus for longer than three months?
Yes
No
Does your cough impact your daily activities?
Yes
No
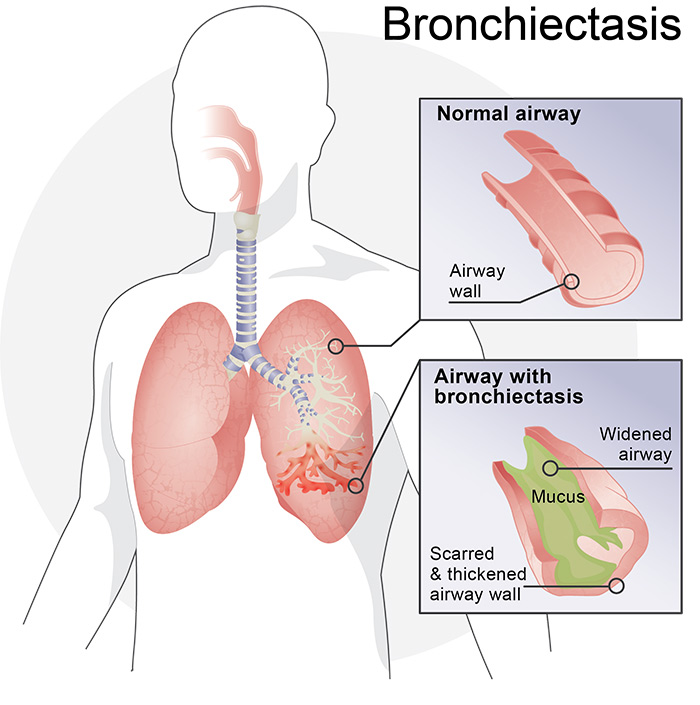
If you answered “yes” to any of the questions, talk with your doctor. Many of the symptoms can be associated with bronchiectasis (BE), a chronic respiratory condition that can produce excess mucus in the lungs that is difficult to cough out. If you have not yet been screened for bronchiectasis, your doctor may order a high resolution CT scan to check for the condition.
Fill out the inquiry form below to receive a BE information packet filled with educational materials to bring to your doctor. Our specialists are always happy to answer any questions you or your physician may have.

800.793.1261
8 a.m. to 5 p.m. CT Monday - Friday